Identify Which of the Following Is Soluble in Water.
That would be copper II hydroxide or CuOH2. I Phenol C6H5OH has the polar group OH and non-polar group C6H5.

Chemical Test For Chlorine 2 Chlorine Chemical Ccls
Identify which of the following substances is soluble or miscible in water a - 5565531 joanespanola449 joanespanola449 25102020 Science Senior High School answered Identify which of the following substances is soluble or miscible in water a polar molecule based on their polarity.

. Thus toluene is insoluble in water. Iii Formic acid HCOOH has the polar group -OH and can form H-bond with water. B Ethylamine is soluble in water due to the formation of hydrogen bonds with water but aniline is insoluble since the large hydrophobic phenyl group does not allow the formation of Hydrogen-bonds.
Views 11K Share. A 3682 g sample of KClO3 is dissolved in enough water to give 375. Identify if the following compound is soluble in water and why.
C_ 6H_ 15 group is of non-polar nature. Nacl is known as sodium chloride It is a ionic compound and soluble in water question_answer Q. Shredded Candle Wax and Water 10Water and Coffee Powder II.
Butanoic acid strongest hydrocarbon butanone butane alcohol carboxylic acid What carboxylic acid is found in each of the following substances. Reset Of the three compounds butanoic acid butane and butanone the one that is most soluble in water is. Thus toluene is insoluble in water.
V Chloroform is water insoluble. Zonrox and Water 5. Ii Toluene C6H5CH3 has no polar groups.
To rank items as equivalent overlap them Reset Help hexane pentanoic acid 1-octanol Most soluble in water Least soluble in water. B From everyday experience you are probably aware that table sugar sucrose C12H22O11 is soluble in water. Iv Ethylene glycol is water soluble.
Glucose is one substance which is completely soluble in water. Is partiallty soluble in water due to weak dipole-dipole interactions in the molecules of pheneol and water. Group the substances in the box provided.
Oil and Water 2. Because of this process sugar is well soluble in water. Soft drinks contain water carbon dioxide and acid due to which iron nail is corroded and appears dull.
Identify the compound in the following group that is most soluble in water Rank compounds from most soluble in water to least soluble in water. Pick one material from the following which is completely soluble in water. Bolivianouft and 1 more users found this answer helpful.
I Phenol is partially soluble in water. Option B is correct. Hence it is soluble in water.
Rank the following substances in order from most soluble in water to least soluble in water. Match the words in the left column to the appropriate blanks in the sentences on the right. C_ 6H_ 5OH.
It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. However halides are soluble in water except those of silver lead and mercury. Efficascent Oil and Water 7.
Answers 1 S Sumit. Thus formic acid is highly soluble in water. Bond breakages need energy.
According to the solubility rules nitrates are soluble in water including the nitrates of silver. Vi Pentanol is partially soluble in water. A Ammonia NH3 is a weak electrolyte.
Thus formic acid is highly soluble in water. 250 ml of 050M HA Ka 30 x 10-7 is titrated with 045M NaOH. Ii Toluene is water insoluble.
Identify a water soluble ointment base from the following products. Powdered juice and Water 9. Oil and Rubbing Alcohol 6.
Leave a Reply Cancel reply. Identify the compound in the following group that is most soluble in water. Your email address will not be published.
Similarly when a salt like sodium chloride dissolves in water the sodium and chloride ions are released and they will interact with polar water molecules. These rules are usually based on experimental observation of diverse groups of compounds. Thus it is highly soluble in water.
Thus phenol is partially soluble in water. Iii Formic acid is water soluble. Thus phenol is partially soluble in water.
Iii Formic acid HCOOH has the polar group OH and can form H-bond with water. Is insoluble in water because it is an aromatic hydrocarbon non-polar while water is polar in nature. A Chalk powder b Tea leaves c Glucose d Sawdust Solution.
The Behavior of Substances in Water Part 1. Ii Toluene C 6 H 5-CH 3 has no polar groups. Required fields are marked.
Fabric conditioner and Water 8. When you do these make sure C1 and C2 are in the same units and V1 and V2 are in the same units. You will need to become familiar with the solubility rules because that will help you determine what compounds are soluble and what compounds are insoluble in water.
C_ 7H_ 18. Ethyl Alcohol and Water 3. Previous Next.
ResetHelp Of the three compounds butanoic acid butane and butanone the one that is most soluble in water is. NaCl is the most soluble because it is a salt. Identify whether the pair of substances are soluble insoluble miscible or immiscible.
Ethanol C 2 H 5 O H forms hydrogen bonding with water. So start with N aCl or sodium chloride. Vinegar and Soy Sauce 4.
Write the balanced chemical reaction for this process including state symbols. Right Answer is. C1V1 C2V2 1ml0001L 01015M 002L C2 0250L C2 000812 Answer is B.
Then propane because both propane and ethane are non polar but propane has more Hydrogen than can bond with water. This energy will be supplied by the formation of hydrogen bonds with water molecules. This is because its functional group can form the intermolecular forces with polar water.
Identify if the following compound is soluble in water and why. Using solubility guidelines which of the following ionic compounds is not properly labeled with respect to solubility in water. 2-butanol because it is an alcohol.
Iv Ethylene glycol has polar -OH group and can form H-bond.

Science Experiments Solubility Of Different Substances Science Experiments Preschool Science Science Experiments Kids
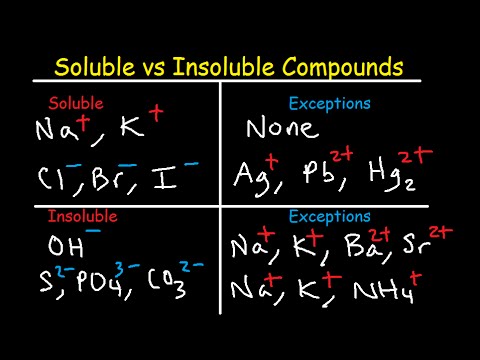
Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube
Comments
Post a Comment